[vc_row full_width=”stretch_row_content_no_spaces” css=”.vc_custom_1661448930417{padding-top: 300px !important;padding-bottom: 220px !important;background: #a3a3a3 url(https://steq.com.br/wp-content/uploads/2022/11/AGLTS_3-1.jpg) !important;}”][vc_column][vc_column_text][/vc_column_text][vc_separator color=”sandy_brown” border_width=”3″ el_width=”10″][/vc_column][/vc_row][vc_row css=”.vc_custom_1564073259210{margin-top: 25px !important;margin-bottom: 25px !important;}”][vc_column width=”3/4″][vc_column_text css=”.vc_custom_1668082692094{padding-bottom: 20px !important;}”]GLOVE LEAK TEST
What does the new revision of Annex 1 of the European standard change for you?
After several years of waiting and several amendments, the European Union recently published the final document revising Annex 1 of Good Manufacturing Practices.
The purpose of the revision of Annex 1 is to reflect the changes that have occurred in the legislation and in the production of sterile pharmaceutical products since the first version in 2007. There are, in fact, many new features contained in the 10 sections that make up the new version of Annex 1, such as glove integrity tests.
INCREASED INSPECTION WITH THE REVISION OF ANNEX 1
The most substantial novelty certainly concerns the definition of the time intervals within which the glove test must be carried out, thus defining an important parameter that was previously left to the discretion of the manufacturer. In fact, the first version of Annex 1, drafted in 2007, states that glove leak tests should occur frequently, but without giving further specifications. The newly published review now specifies that glove integrity tests on insulators should be performed at set intervals, at least at the beginning and end of each production batch.
“Tests should be carried out at defined intervals.
In general, glove integrity tests should be carried out at a minimum frequency at the beginning and end of each batch or campaign.”
In the case of very small productions processed by hand, the criterion is no longer the batch, but the manufacturing session.
“For manual aseptic processing activities, where small batches are produced, the frequency of integrity check may be based on other criteria,
as the beginning and end of each manufacturing session.”
From August 25, 2023, the new Annex 1 will come into force and all pharmaceutical companies in the European territory, or with the intention of marketing their products in this market, must adhere to the new guidelines and increase the frequency of their tests on gloves. The technology and methodology used to conduct these glove tests will play a crucial role in the outcome and effectiveness of each test.

EQUIPMENT FOR CARRYING OUT THE TESTS
With the increase in the number of tests due to the production of batches, it is even more crucial to avoid the risk of encountering both false positives and false negatives. In both cases, the result would certainly be the interruption of production until the problem was identified and resolved. In the worst case, it may even be necessary to destroy the entire batch in question. In this sense, thoroughly evaluating the reliability of a piece of equipment to carry out the tests before purchase could avoid possible future problems.
EQUIPMENT CONFIGURATION AFFECTS THE SPEED OF TESTING
There is no doubt that the speed of the equipment during testing plays a key role in the efficiency of the production cycle. But this is not the only parameter that must be taken into account: the setup time of the instrument is also important and helps to simplify the overall test procedure.
OUR EQUIPMENT
The AGLTS (Automatic Glove Leak Testing System) from our represented TEMA Sinergie, stands out both for the ease of configuration of the instrument and for the speed of testing to make the testing procedure simple, fast and easy.
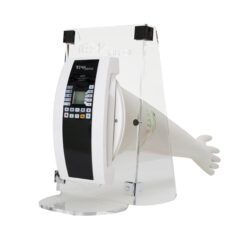
An important feature of AGLTS is the ergonomics and ease of use of the equipment. The ease of use of AGLTS is defined by preloaded standard test methods, as well as customizable methods, quick start button, and several other management options, compliant with the 21 CFR Part 11 standard. AGLTS is definitely the most advanced integrity testing system for Isolators and RABS in the pharmaceutical industry. As it is an instrument used almost exclusively in hazardous environments, the AGLTS is manufactured with materials that are resistant to sanitizing agents and bio-decontamination processes.
To learn more, contact us at contato@steq.com.br or (11) 5186-9400.[/vc_column_text][/vc_column][vc_column width=”1/4″][vc_basic_grid post_type=”post” max_items=”3″ element_width=”12″ gap=”15″ item=”958″ grid_id=”vc_gid:1668082664836-ca7df5cbf3757f055dc5a44dc76311e5-5″ taxonomies=”4, 1, 5, 3″][/vc_column][/vc_row]




